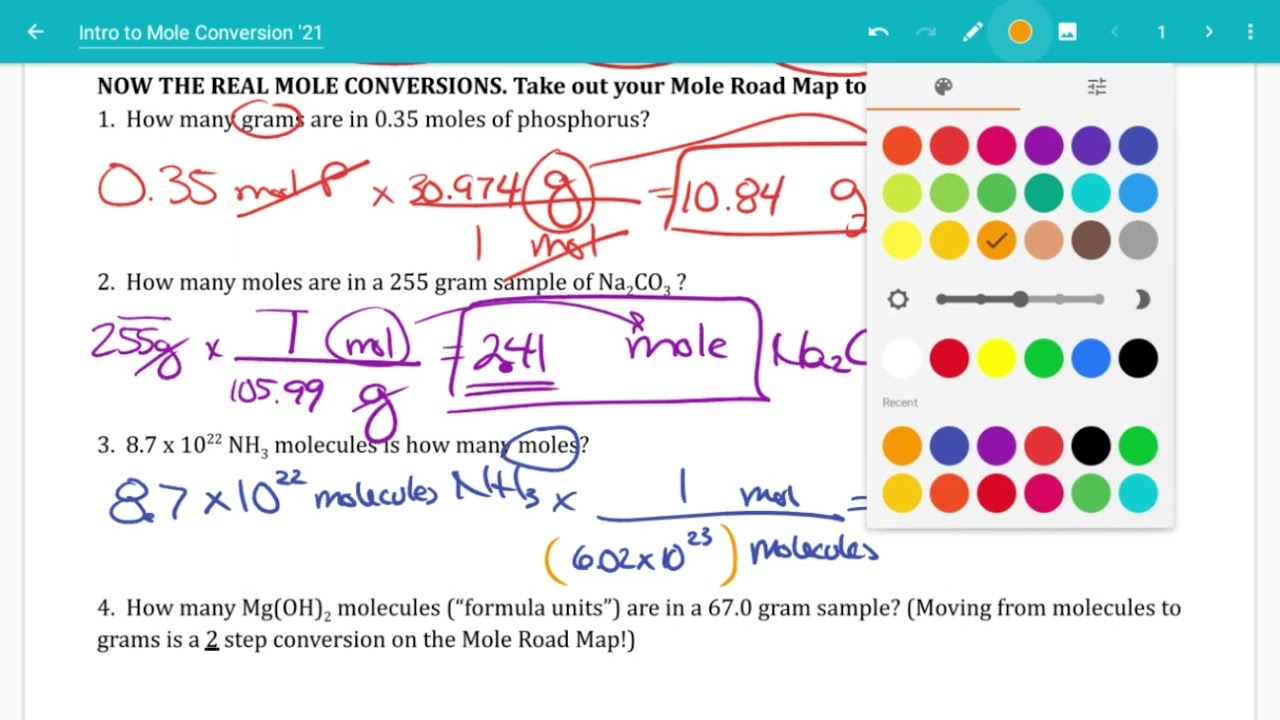
Understanding mole conversions is a fundamental concept in chemistry. Mole conversions involve converting between the mass, number of particles, and volume of a substance. This skill is essential for solving various problems in chemistry, such as determining the amount of reactants needed for a chemical reaction or the products formed.
It is crucial for students to practice mole conversions through worksheets to reinforce their understanding of the concept. These worksheets typically consist of problems that require students to convert between moles, grams, molecules, and volume using conversion factors and the mole concept.
Practice Problems
Here are a few sample problems that you might encounter in a mole conversions worksheet:
1. Convert 25.0 grams of H2O to moles.
2. Determine the number of molecules in 2.0 moles of CO2.
3. Convert 500.0 mL of HCl to moles.
4. Calculate the mass of 3.0 moles of NaCl.
5. Convert 0.75 moles of O2 to liters at STP.
These problems require students to apply the mole concept and conversion factors to arrive at the correct answers. By practicing these problems, students can improve their problem-solving skills and gain confidence in their ability to work with mole conversions.
It is essential for students to show their work and units in these problems to ensure they are following the correct steps and conversions. This practice will help reinforce the steps needed to convert between different units and quantities accurately.
Additionally, students can check their answers using online resources or textbooks that provide solutions to mole conversion problems. This feedback can help students identify any mistakes and learn from them to improve their understanding of the concept.
Overall, practicing mole conversions through worksheets is crucial for mastering this fundamental concept in chemistry. By working through various problems and applying the mole concept, students can develop their problem-solving skills and gain confidence in their ability to work with mole conversions effectively.